Reverse osmosis (RO) technology is widely used in the water treatment industry, especially in seawater desalination, drinking water purification, and industrial water treatment fields. The reverse osmosis membrane is the core component of this process. It selectively allows water molecules to pass through while retaining dissolved salts, organic matter, colloids, and other impurities. However, as the system operates, membrane surface fouling inevitably occurs, leading to a decline in membrane flux, increased system energy consumption, and deterioration of the effluent water quality. To maintain stable system operation,online cleaning (CIP) has become an effective maintenance method.
Main Factors of Membrane Fouling
- Convective Deposition
In a reverse osmosis system, as water passes through the membrane under pressure, dissolved substances (such as salts, colloids, microorganisms, organic matter, etc.) and suspended particles are retained by the membrane. During this process, the convective movement of water on the membrane surface causes these retained substances to gradually deposit on the membrane surface. This substance deposition caused by convection is called convective deposition. It is the main cause of reverse osmosis membrane fouling.
- Concentration Polarization
Concentration polarization refers to the phenomenon where, during the separation process, the solution in the feed liquid passes through the membrane under pressure, and solutes (ions or solutes of different molecular weights) are retained, causing the concentration at the membrane and bulk solution interface or near the membrane interface region to increase. Under the influence of the concentration gradient, the solutes will diffuse from the membrane surface to the bulk solution, forming a boundary layer, which increases fluid resistance and local osmotic pressure, thereby leading to a decrease in solvent permeation flux. Concentration polarization accelerates membrane fouling. Due to concentration polarization, the solute concentration near the membrane surface increases rapidly, causing an increase in boundary layer fluid resistance (or local osmotic pressure), leading to a decrease in mass transfer driving force and resulting in fouling deposition.
- Retention of intercepted substances
The intercepted substances accelerate membrane fouling. For example, in spiral-wound membranes and flat-sheet membranes, there is a layer of plastic mesh between the feed channels, which supports the membrane and increases turbulence, but also causes interception. The pollutants are blocked by the mesh and quickly deposit.
Under What Circumstances Is Cleaning Required?
The reverse osmosis system needs to be cleaned before the membrane fouling becomes severe, as this will increase the difficulty of cleaning. The reverse osmosis membrane should be cleaned immediately when the following conditions occur during operation:
- Under normal feed water pressure, the permeate flow decreases by 10%–15% from the normal value;
- To maintain normal permeate flow, the feed water pressure increases by 10%–15% after temperature correction;
- The permeate water quality decreases by 10%–15%, and the salt passage increases by 10%–15%;
- The pressure differential between different stages of the system increases significantly.
How to Perform Online Cleaning?
Online cleaning is a method of cleaning membrane elements without disassembly, typically including the following steps:
Contaminant analysis
Common reverse osmosis contaminants include inorganic scale, metal oxides, acid-insoluble scale, silica scale, etc. To effectively address these different contaminants, appropriate cleaning methods must be employed. Incorrect selection of cleaning chemicals and methods can sometimes exacerbate membrane system contamination. Therefore, it is necessary to determine the type of fouling on the membrane surface before cleaning. The following analysis methods are available:
- Conduct a loss on ignition test to calculate the ratio of organic to inorganic matter in the pollutants based on the weight change before and after burning, to determine the type of pollution.
- Analyze the types and contents of elements using X-ray fluorescence spectroscopy (XRF) and X-ray diffraction spectroscopy (XRD) to determine the main components of inorganic pollutants.
- Combine Fourier transform infrared spectroscopy (FTIR) and gas chromatography-mass spectrometry (GC-MS) to characterize and identify the chemical species of pollutants, thereby obtaining information on the organic composition of membrane pollutants.
Selection of Cleaning Solution
The type of cleaning solution is determined based on the analysis of pollutants present before cleaning. To achieve optimal cleaning results, sometimes a combination of different chemical cleaning agents is used.
Acidic cleaning solutions can be used to remove inorganic scale deposited on the reverse osmosis membrane, while alkaline cleaning solutions can be used to remove organic matter and colloidal contaminants. The following are some common chemical cleaning agent selection schemes:
| Pollutants |
Preferred Chemical Cleaning Agent |
Cleaning Conditions |
Alternative Chemical Cleaning Solution |
| Inorganic scale |
0.2% HCL solution |
PH: 1–2; temperature < 38 °C |
2% citric acid; 1% |
| Metal oxides |
1% solution |
Temperature < 35 °C |
0.5% phosphoric acid; 2% citric acid |
| Acid-insoluble scale (CaF2, BaSO4) |
0.1% NaOH + 1% Na4 EDTA (ethylenediaminetetraacetic acid tetrasodium salt) |
PH: 11–12; temperature < 30 °C |
1% sodium hexametaphosphate |
| Silica scale |
0.1% NaOH solution + 0.025% Na-SDS (sodium dodecyl sulfate) |
PH: 11–12; temperature < 30 °C |
0.1% NaOH + 1% Na4 EDTA solution |
| Microorganism |
0.1% NaOH+ 0.025% Na-SDS |
PH: 11–12; temperature < 30 °C |
0.1% NaOH + 1% Na4 EDTA solution |
| Organic matter |
0.1% NaOH solution + 0.025% Na-SDS, used as the first step of cleaning |
PH: 11–12; temperature < 30 °C |
0. 2% HCL solution is usually used as the second step of cleaning after alkaline washing |
Calculation of Cleaning Solution Volume
There are two methods to calculate the volume of cleaning solution for the reverse osmosis system:
Cleaning Process
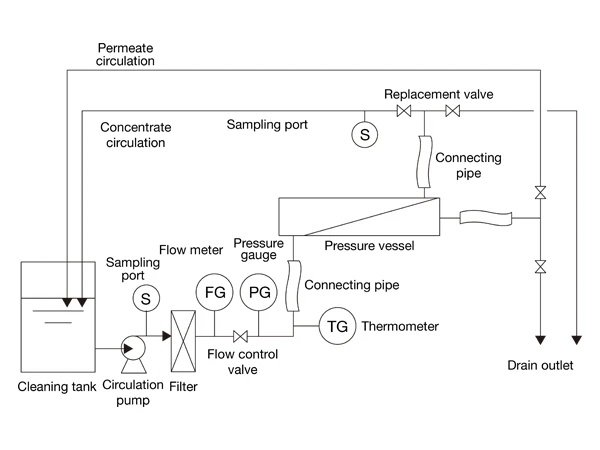
Online cleaning is performed with the membrane elements retained in the pressure vessel. Cleaning equipment generally includes cleaning tank filters, circulation pumps, pressure gauges, thermometers, pressure gauges, valves, sampling points, and pipelines. The volume of the cleaning tank should ensure that it meets the requirements for the amount of water used to replace the water in the connecting hoses, pipelines, and reverse osmosis pressure vessels.
- Formulation of the cleaning plan
① In this article, we use a combination of alkaline and acidic cleaning agents to clean the reverse osmosis membrane system, which contains CaF2 and inorganic scale. Hydrochloric acid and sodium hydroxide solutions are used to adjust the pH value of the cleaning solvent.
- Preparatory work before cleaning
② Open the inlet valve of the reverse osmosis device and inject a certain amount of permeate into the reverse osmosis cleaning tank. Start the reverse osmosis circulation pump to circulate and clean the reverse osmosis cleaning pipeline, ensuring the pipeline is clean and free of debris.
③ Turn off the reverse osmosis circulation pump, empty the reverse osmosis cleaning tank, and inject reverse osmosis permeate to a certain height (considering the entire pipeline's capacity). Start the heater to heat the water in the cleaning tank to about 40 °C.
④ Prepare hydrochloric acid and sodium hydroxide solutions to adjust the pH value of the cleaning solvent. Prepare a pH meter, measure and record the changes in the pH value of the cleaning solution during the cleaning process.
- Prepare the alkaline cleaning solution
Start the reverse osmosis circulation pump for circulation, add the alkaline cleaning agent into the cleaning tank, then add an appropriate amount of alkali (NaOH) solution, and measure the pH value of the solution to ensure it reaches around 11.
- Alkaline cleaning of reverse osmosis membrane
① Reverse osmosis alkaline cleaning: Adjust the reverse osmosis circulation pump to maintain the outlet pressure at around 0.2–0.25 Mpa. Allow the reverse osmosis alkaline cleaning agent to circulate within the reverse osmosis system. Measure the pH value of the alkaline solution every 10 minutes. If it decreases, it indicates that contaminants on the membrane surface are being dissolved by the cleaning solution. Add an appropriate amount of alkaline (NaOH) solution to maintain the pH value of the solution around 11. Continue circulating until the pH value of the cleaning solution no longer changes (generally, it needs to circulate for more than 30 minutes).
② Soaking with alkaline cleaning agent: After the reverse osmosis membrane has been cleaned with the alkaline solution, stop the reverse osmosis circulation pump and close all valves of the reverse osmosis system, allowing the reverse osmosis membrane to be soaked in the alkaline solution for a certain period (generally more than 5 hours).
③ Recirculation of the alkaline cleaning agent: Open the reverse osmosis device cleaning inlet valve, outlet valve, and permeate side return valve to allow the alkaline cleaning agent to flow through the membrane in series. Record any changes in the solution's pH value (typically recirculate for more than 1 hour).
④ Rinse the alkaline solution thoroughly: Turn off the reverse osmosis circulation pump and close the outlet valve. Open the cleaning tank drain valve to empty the reverse osmosis alkaline cleaning solution. Close the reverse osmosis device cleaning inlet valve and outlet valve. Open the concentrate discharge valve and permeate side drain valve to empty the alkaline cleaning agent from the reverse osmosis device. Then open the flushing inlet valve and start the reverse osmosis flushing pump to flush the reverse osmosis system. Once the conductivity of the reverse osmosis permeate is below 30 μs/cm, open the reverse osmosis cleaning inlet valve and cleaning outlet valve respectively to backwash the pipeline and cleaning tank until the alkaline cleaning agent in the pipeline is completely flushed out.
- Prepare acidic cleaning solution
Inject a certain height of reverse osmosis permeate into the reverse osmosis cleaning tank. Open the reverse osmosis cleaning pump circulation valve, start the reverse osmosis circulation pump for circulation, add an appropriate amount of acidic cleaning agent and hydrochloric acid solution until the solution pH value reaches 2.0.
- Cleaning and soaking, rinsing with acidic cleaning solution
The acid washing process of the reverse osmosis membrane is basically the same as the alkaline process. During the cleaning process, the pH value of the solution needs to be frequently tested. If the pH value rises, it indicates that there are deposits neutralizing with the acidic cleaning agent. It is necessary to add an appropriate amount of hydrochloric acid solution to maintain the cleaning solution at around 2.0. After cleaning, it is also necessary to be soaked for more than 5 hours, and then circulate again for 1 hour, and record the pH value changes. Then start the entire reverse osmosis cleaning system to rinse the reverse osmosis device clean.
- Restart the system
After the components and system have stabilized, record the operating parameters after the system restarts.
Online Cleaning Precautions
- The cleaning solution temperature should be maintained within 25–35 °C. The cleaning solution must be prepared with reverse osmosis permeate or deionized water, and the solution should be mixed uniformly.
- During cleaning, the residual water in the reverse osmosis pressure vessel and pipelines should be drained, and the concentrate generated during the cleaning process should be circulated back to the cleaning solution tank. If the returned cleaning solution becomes noticeably discolored or turbid, a new cleaning solution should be prepared. If the pH change of the recirculated cleaning solution exceeds 0.5, the pH should be readjusted or the cleaning solution should be replaced.
- After the cleaning is completed according to the specified time, it should be immediately rinsed with reverse osmosis permeate or deionized water. The rinsing time is generally 30–60 minutes, and the reverse osmosis outlet water should be drained. After confirming the rinsing is completed, it is best to put it into normal operation immediately. The time from chemical cleaning to operation should not exceed 24 hours.
- Cleaning solution preparation personnel should pay attention to safety, wear protective gloves, glasses, and protective clothing, and prepare emergency water and emergency medicines on site.
- Instruments and pipeline valves unrelated to the cleaning work should be closed to ensure effective isolation.