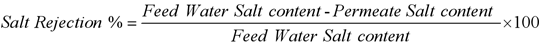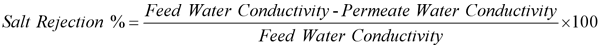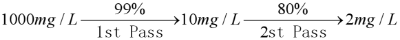Calculation & Influencing Factors of Salt Rejection
What Is the System Salt Rejection?
The salt rejection of a RO system is an important indicator of the reverse osmosis process, which refers to the efficiency of the membrane element in removing dissolved solids from the water entering the system during the reverse osmosis process, usually expressed as a percentage. The higher the reverse osmosis salt rejection, the better the effect of the system on the removal of impurities in the water, the better the water quality. Efficient reverse osmosis desalination technology is widely used in areas such as desalination and industrial water treatment.
RO system salt rejection calculation
Salt rejection refers to the RO membrane's ability to allow the solution to pass through while preventing dissolved solids from passing through, the total dissolved solids (TDS) in the liquid is simply the salt content of the liquid, and the TDS value is proportional to the electrical conductivity. There are two methods to calculate salt rejection:
Main Influencing Factors of Salt Rejection
- Feed water pressure
Elevated pressure of the feed water will increase the water flux of the RO membrane, and salt rejection will also increase to a certain extent. When it reaches a certain degree of salt rejection, the salt rejection will not change, and more than a certain value will damage the membrane element.
- Feed water temperature
RO membrane is very sensitive to changes in feed water temperature. Feed water temperature directly affects water viscosity and salt movement rate. As the temperature increases, the salt transmission rate increases, the electrical conductivity of the permeate water side increases, and salt rejection will be reduced. That is, feed water temperature increases, membrane salt rejection decreases, and vice versa, membrane salt rejection is higher at low temperatures.
- Feed water salt concentration
When the feed water pressure is unchanged, the higher the salt content of the feed water, the higher the osmotic pressure, the membrane salt permeability will also rise, and the more salt permeation and salt rejection will fall.
- Inlet water pH
The RO membrane has the highest salt rejection at a pH of about 7. The salt permeability of the membrane system varies with pH, but too high or too low a pH can cause damage to the membrane.
- Incomplete pre-filter for impurity removal
If the pre-filter system before entering the RO membrane to remove impurities is not good, too many impurities in the RO membrane will cause clogging and contamination of the RO membrane, affecting the filtration performance of the membrane, resulting in poor quality of permeate water and lowering the rejection rate.
- Recovery rate
A high recovery rate means that more water is recovered and reused, and the concentration of solutes in the water will gradually increase, resulting in a lower rejection rate.
- Ion valence and salt molecule size
The salt rejection of the osmosis membrane for different substances is mainly determined by the structure and molecular weight of the substances, the salt rejection for high valence ions and complex monovalent ions is higher, and the salt rejection for monovalent ions, such as sodium ion, potassium ion, chloride ion, is slightly lower, and the salt rejection will increase with increasing molecular diameter.
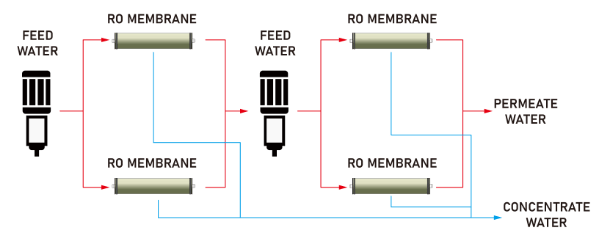
- Higher salt rejection in 2nd pass RO system than in 1st pass RO system
2 pass RO system means that the permeate water from the 1st pass process becomes feed water to the 2nd pass inlet. The salinity of the 1st pass permeate water is already relatively low, and then the 1st pass permeate water is filtered through the RO membrane. Although the salt rejection of the 2nd pass will be much lower than that of the 1st pass, the total salt rejection of the system will be increased.
For example:
Assuming that the TDS value of the water is 1000 mg/L, the salt rejection of the 1st pass RO membrane is 99%, and the 2nd pass salt rejection is 80%, at this time, the whole system salt rejection reaches (1000 - 2) / 1000 = 0.998 = 99.8%, which is higher than the 99% salt rejection of the 1st pass RO membrane.
How to Improve the Salt Rejection?
- Adjust the pH value and temperature environment suitable for the membrane element to work, and find the maximum working efficiency of the membrane element;
- Do not pursue too high a recovery rate, resulting in a decrease in permeate water quality and a reduction in salt rejection; determine the appropriate balance of recovery rate and salt rejection according to specific requirements and water quality characteristics;
- Improve salt rejection by appropriately increasing operating pressure;
- Regular cleaning and maintenance procedures to minimize contamination and fouling of the membrane surface;
- To avoid problems such as clogging and contamination of membrane elements that may affect salt rejection, an additional pre-filter system needs to be installed in the early stages to remove as many impurities as possible and keep RO membranes operating efficiently;
- If the salt rejection of the 1st pass reverse osmosis process fails to achieve the desired effect, a 2nd pass reverse osmosis system can be added, and the whole system salt rejection will be improved.