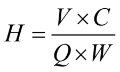Ion exchange resin is a polymer compound insoluble in acids, alkaline solutions, and organic solvents. It has high physical and chemical stability and is an important material used in water treatment, chemical separation, biological separation, and other fields. Its principle is to use the ionic functional groups on the resin to react chemically with the ions in solution, thus achieving the separation and purification of ions.
Structure
The elements that make up an ion exchange resin are generally carbon, hydrogen, oxygen, nitrogen, and sulfur. The constituent units are the polymer backbone, functional groups attached to the backbone and exchangeable ions in the functional groups. The polymer backbone is a three-dimensional multidimensional mesh structure with interconnected and entangled polymer chains, not involved in ion exchange reactions, and the chains are charged functional groups with ions of opposite charge called exchangeable ions or counter ions. The polymer backbone and functional groups cannot move freely, but counter ions can dissociate into freely moving ions in the solution. The ability to exchange with other counter ions of the same charge from the outside under certain conditions and the process of dissociation or resolution is reversible, which determines the ion exchange performance of the resin.
Formation process and chemical structure of strong acid cation exchange resin
Why We Need Resin?
Excessive water hardness will cause a lot of inconvenience for many industries. For example, in domestic water, excessive water hardness will reduce the taste of drinking water, and in serious cases will affect human health. In boiler water supply, hardness ions in the water will also generate boiler scale in the boiler, which not only wastes fuel, but also causes explosions. In the field of seawater desalination, there are not only a large number of Na+, K+, Cl-, CO32-, and SO42-, but also a high concentration of Ca2+ and Mg2+, without any treatment, will precipitate a large number of precipitates and cause irreversible scaling inside the system, reducing the recovery rate of water and the stability of operation, thereby increasing operating costs. Therefore, whether from the perspective of domestic water safety or desalination system anti-fouling and scale inhibition, it is inevitable to control the water hardness within a certain range.
Ion Resin Softening & Regeneration Process
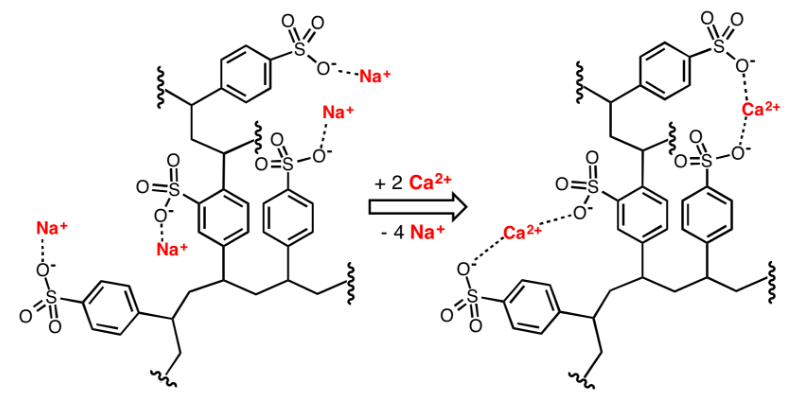
The exchangeable ions carried on the cation resin used for water softening are generally Na+ and H+, which can react with Ca2+ and Mg2+ in the solution. Na-type cation exchange resin, for example, in the solution at room temperature and low concentration, due to the stronger affinity between Ca2+ (or Mg2+) and cation exchange resin, the reaction to the right, Na+ in the resin is continuously replaced by Ca2+ (or Mg2+) until the reaction equilibrium; when the concentration of Na+ in the solution is greater (i.e., the resin is added to saturated saline or HCl solution), the whole reaction is carried out to the point where the concentration of Na+ in the solution is greater (i.e., saturated saline or HCl solution is added to the resin), the whole reaction will proceed to the left, i.e., Ca2+ (or Mg2+) in the resin is continuously desorbed to regenerate the ion exchange resin.
Softening and regeneration of Na+ exchange resin
Resin Regeneration Cycle Calculation
The resin regeneration cycle refers to the time used when the exchange resin gradually loses its adsorption capacity after a period of use and reaches the saturant state. The regeneration cycle is influenced by many factors such as water flow, total water hardness, resin selection, etc.
According to the volume of the water softener tank, calculate the volume of resin filling, which is generally 60% to 90% of the height of the water softener tank.
We can find the regeneration cycle as follows: 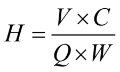
Periodic water production can also be obtained as: 
- H — Regeneration cycle (hour)
- Qc — Periodic water production (m3)
- V — Resin volume (m3)
- C — Working exchange capacity (mmol/L)
- Q — Inlet flow rate (m3/h)
- W — Total water hardness (mmol/L)
For example, when Q = 40 m3/h; V = 2 m3; C = 800 mmol/L; W = 5 mmol/L, then H = 2 × 800 ÷ (40 × 5) = 8 hour, and Qc = 2 × 800 ÷ 5 = 320 m3.
Recycled Salt Consumption
The resin needs to be regenerated with the corresponding salt, acid, and alkali after failure to restore its working capacity, and the regenerant consumption and regenerant ratio are generally used to measure the regeneration capacity of the resin. Regenerant consumption = Regenerant ratio × Molar mass, so Cycle salt consumption = Regenerant ratio × Molar mass (g/mol) × Working exchange capacity (mol/L) × Resin filling volume (L).