
Scaled pipe

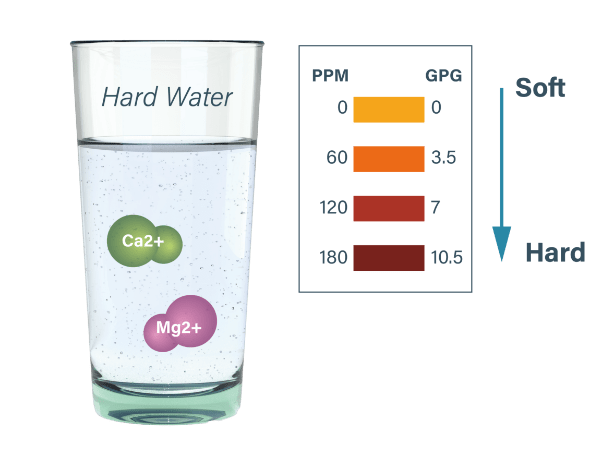
Water hardness refers to the content of calcium and magnesium in water. According to the value of water hardness, water can be roughly divided in to hard water and soft water. Hard water contains a large amount of calcium and magnesium ions, and may cause pipe scaling, RO membrane fouling and blockage or reduced equipment efficiency. The standards for water hardness vary in different regions. The United States Geological Survey (USGS) has the following criteria for classifying water quality as follows.

Scaled pipe

Scaled cup
Hardness is also divided into temporary hardness and permanent hardness.
Temporary hardness is composed of calcium and magnesium ions and bicarbonate. Bicarbonate is unstable and can be removed by boiling.
Permanent hardness is composed of calcium and magnesium ions and sulfate, nitrate, chloride, etc. These ions cannot be removed by boiling and requires water treatment processes like ion exchange softening to remove.
Water hardness refers to the ability of water to form precipitates, and the ions that cause precipitation are mainly Ca2+ and Mg2+, so the water hardness is basically equal to the sum of the calcium hardness and magnesium hardness. Based on the molar mass of calcium and magnesium ions, the total hardness can be calculated by the following formula:
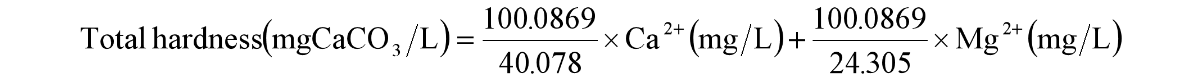

To save your valuable time, you may use total water hardness calculator directly. If your calculation result is greater than 60 mg CaCO3/L, it indicates that the water quality is hard and you may consider to purchase a softener to softening your water quality.
The unit of water hardness is typically expressed in mEq/L, which means the the number of milliequivalents per liter of solution, but now the international flow hardness unit is mmol/L. Germany often uses °dH (German degree), British commonly uses °e (English degree) and France typically use°f (French degree) as the hardness units of their own countries. Besides, there are also ppm CaCO3, mg CaCO3/L, gpg (grains per gallon), and the hardness unit conversion chart is shown below.
To save your valuable time, we provide you with a basic units converter
| mmol/L | mEq/L | gpg | US | Germany | UK | France | ||
|---|---|---|---|---|---|---|---|---|
| ppm CaCO3 | °dH | °e | °f | |||||
| 1mmol/L | 1 | 1.99998 | 5.84689 | 100.0869 | 5.60774 | 6.99909 | 10.0087 | |
| 1mEq/L | 0.5 | 1 | 2.92347 | 50.044 | 2.8039 | 3.49958 | 5.0044 | |
| 1gpg | 0.17103 | 0.34206 | 1 | 17.118 | 0.9591 | 1.19706 | 1.7118 | |
| US | 1 ppm CaCO3 | 0.00999 | 0.01998 | 0.05842 | 1 | 0.05603 | 0.06993 | 0.1 |
| Germany | 1 °dH | 0.17833 | 0.35665 | 1.04265 | 17.848 | 1 | 1.24811 | 1.7848 |
| UK | 1 °e | 0.14288 | 0.28575 | 0.83538 | 14.3 | 0.80121 | 1 | 1.43 |
| France | 1 °f | 0.09991 | 0.19982 | 0.58418 | 10 | 0.56029 | 0.69930 | 1 |